
Export to USA

Conditions for the export of pork and beef from the EU to the USA
The authorities responsible for the import of food, including meat, for the US market are: the FDA (U.S. Food & Drug Administration), and the FSIS (Food Safety and Inspection Service), an organizational unit of the USDA – (United States Department of Agriculture).
The rules regarding production, production supervision and export of food to the USA have been included in a number of legal acts, including the Federal Meat Inspection Act (MIA), the Law on Modernization of Food Safety (Modernization Act), as well as in the relevant FSIS and Federal Code of Conduct Regulations (CFR).
Below is a link to the FSIS website, where you can read the relevant regulations in detail:
https://www.fsis.usda.gov/wps/portal/fsis/topics/regulations

Before beginning export[1]
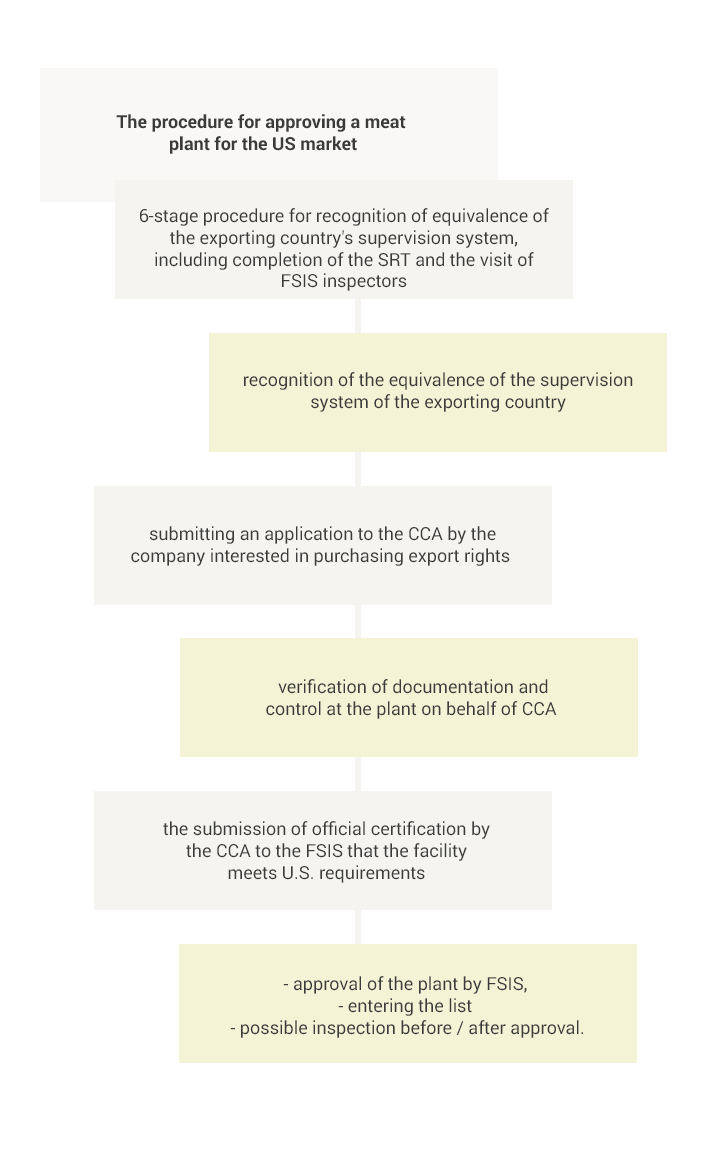
The export of meat and its products to the USA is only possible from countries whose food safety supervision system has been recognized as equivalent to the US system and only from establishments approved by the competent veterinary services and listed on the specific list.
The equivalence of a given country is determined by the FSIS on the basis of a 6-step procedure, the two most important elements being an assessment of the extensive documentation provided by the central authorities (CCA) of the applying state (the so-called SRT-Self Reporting Tool) and then the on-site visit to verify the information contained in the application.
After official recognition of the equivalence of the procedures used in a given country, the FSIS relies on control activities carried out by the CCA in that country. Therefore, a company interested in purchasing export licenses for the US market submits a relevant application to the CCA. Then, after proper verification, the CCA provides FSIS with official certification that the entity meets the US requirements. In addition, the FSIS may carry out additional on-site inspection before or after approval of the establishment to ensure that the food safety system remains equally valid at the company level.
https://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-products/eligible-countries-products-foreign-establishments/eligible-foreign-establishments
[1] Source: FSIS website: www.fsis.usda.gov
Listy zakładów posiadających uprawnienia do eksportu na rynek USA są dostępne na stronach internetowych właściwych władz weterynaryjnych państw członkowskich UE oraz na stronie FSIS pod następującym linkiem:
https://www.fsis.usda.gov/wps/portal/fsis/topics/internation-al-affairs/importing-products/eligible-countries-products-foreign-establishments/eligible-foreign-establishments
The requirement of additional registration of enterprises[2]
The Public Health Security and Bioterrorism Preparedness and Response Act 2002 requires establishments producing, storing or otherwise associated with food for sale in the United States to be registered with the FDA. Registration is free and takes place via e-mail, fax or electronic system. Information and tips on how to register a plant can be found on the FDA website dedicated to this topic at the following address:
https://www.access.fda.gov/
Registration is valid for two years. It should be noted that for each registered foreign plant, a representative established in the United States should be provided.
[2] The information comes from the FDA website: www.fda.gov

General rules for food companies:
US food law requires that food products imported into and sold in the United States do not carry or contain any toxic or harmful substances that may make them harmful to health, and that they do not consist wholly or partially of contaminated substances, rotten or decaying substances, or in any other way unfit for consumption.
Manufacturers do not need to provide the FDA with any test results as a condition for production, marketing and distribution of food products, and the FDA does not accept any food samples from producers, importers and distributors.
Meat plants should have operational procedures related to sanitary standards (so-called SSOP) in accordance with the requirements of Title 9 of the Federal Regulations Code (CFR).
In addition, exporting establishments should apply the current US Food GMPs (Good Manufacturing Practices) for food products. These practices define the basic factors that manufacturers and distributors should take into account to ensure cleanliness and food safety during its production, processing, packaging and storage. Good Manufacturing Practices can be found in Part 110 of Title 21 CFR.
The FDA regulates the issue of additives used in food products. New food additives must be approved or inspected by the FDA prior to being placed on the market. The FDA also regulates food packaging materials and substances in contact with food. Packaging materials must be safe to use and must not transfer harmful substances or stale flavors and fragrances to the packaged product.
The list of food additives approved by the FDA is included in the list available at the following link:
https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm091048.htm
Bulk food containers imported into the United States should have the following information in English on the outside of the container: product designation, name and address or telephone number of the company (which may be the manufacturer, distributor, importer, importing agent or commissionaire), net weight in English measures (pounds/units), list of ingredients contained in the product and country of origin of the product. Labels are subject to approval by the FSIS
In the case of foods known as 'low-acid canned foods' (LACF) or 'acidified foods' (AF), it is subject to special requirements, and plants exporting such products, to additional registration in the FDA.
Specific requirements for the export of pork and beef
The US veterinary authorities draw attention to the following animal diseases during import of meat and its products: rinderpest, foot-and-mouth disease (FMD), swine vesicular disease (SVD), African swine fever (ASF), and classical swine fever (CSF).
The USA, as a member of the World Organization for Animal Health (OIE), respects the principles of regionalization in the case of specific infectious animal diseases, including FMD, SVD, ASF and CSF.
In the case of products containing raw beef, producers must have implemented test procedures for E. coli O157H7 sticks.
Also, in the case of meat plants producing ready-to-eat foods (RTE), it is necessary to implement an appropriate methodology for testing Listeria monocytogenes.
Meat exported to the US is subject to customs duties. The current amount of duties is available on the website of the European Commission:
Pork:
http://madb.europa.eu/madb/atDutyOverviewPubli.htm?countries=US&hscode;=0203
Beef:
http://madb.europa.eu/madb/atDutyOverviewPubli.htm?countries=US&hscode;=0202